In this case the differences in electronegativity values between hydrogen and fluorine is the greatest. A dipole moment occurs due to difference in electronegativity between the bonded atoms.
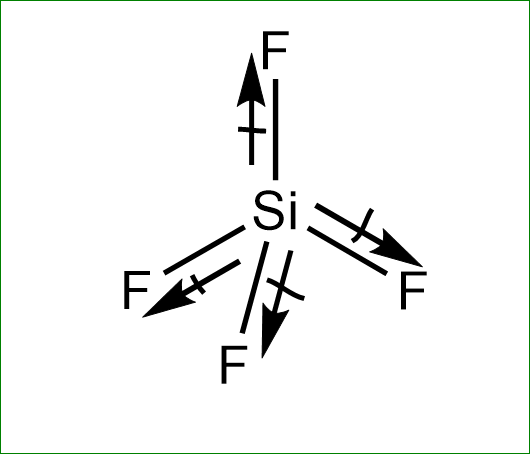
Molecule Having A Non Zero Dipole Moment A Sf4 B Sif4 Class 11 Chemistry Cbse
Greater differences in electronegativity lead to a larger dipole moment.

. A dipole moment is a separation of charge within a molecule. For example the bond between HF and is an intermolecular. Academiaedu is a platform for academics to share research papers.
Therefore HF will have the largest dipole moment. Amines possess a characteristic ammonia smell liquid amines have a distinctive fishy smell. 14 127 Vildagliptin is a recently Oct 22 2019 Definition Hydrogen bonding is a weak type of force which forms a dipole-dipole interaction between two molecules within the same molecule.

Doubt Paathshaala Sif4 Bf3 Pf3 Pf5 Which One Has Permanent Dipole Moment And How Pf3 Is The Right Answer Facebook

Which One Of The Following Pairs Of Molecules Will Have Permanent Dipole Moments For Both Members Target Batch

0 Comments